NeoMatriX ® Wound Matrix
New Skin in The Game™

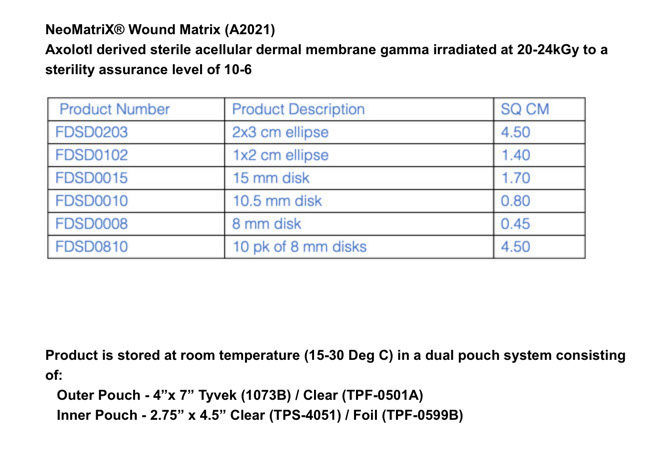
About NeoMatriX®
NeoMatriX® Wound Matrix is fabricated from the dermal extracellular matrix of axolotl. NeoMatriX® is provided as sheets of various sizes for placement on wound beds to help manage the wound environment.
Indications for Use
NeoMatriX® Wound Matrix is intended for the management of wounds including:
This device is terminally sterilized using gamma irradiation and is intended for one-time use.
• Partial and full-thickness wounds
• Pressure ulcers
• Venous ulcers
• Diabetic ulcers
• Chronic vascular ulcers
• Tunneled/undermined wounds
• Surgical wounds (donor sites/grafts
post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence)
• Trauma wounds (abrasions, lacerations, partial thickness burns, skin tears)
• Draining wounds
Biocompatibility & safety Testing
NeoMatriX® has been rigorously tested in accordance with recognized biocompatibility standards
• Cytotoxicity
• Sensitization
• Irritation/intracutaneous reactivity
• Acute systemic toxicity
• Pyrogenicity
• Subacute and sub-chronic toxicity
• Genotoxicity
• Endotoxin testing was conducted according to AAMI ST72
• Sterilization validation using gamma radiation to a sterility assurance level of 10-6
• Viral inactivation testing was also performed
• Pre-clinical testing showing non-inferiority in a porcine wound healing model
• Clinical testing indicating that NeoMatriX Wound Matrix does not raise aimmunogenicity concerns when used in humans

Address
13800 Tech City circle, Ste. 200,
Alachua, FL 32615
Copyright 2019. All Right Reserved / MKT-001 Rev 0 / Designed By SPG



